Efficacy, Safety, and Tolerability of Centanafadine Sustained-release Tablets in Adults With ADHD: Results of Two Phase 3, Randomized, Double-blind, Multicenter, Placebo-controlled Trials
Lenard A. Adler, MD1; Julie Adams, MD2; Jessica Madera, MD2; Mary Hobart, PhD2; Denise Chang, PhD2; Mark Angelicola, MS2; Robert McQuade, PhD2; Michael Liebowitz, MD3
1NYU Langone Health, New York, NY; 2Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ;
3The Medical Research Network, LLC, New York, NY
These edited and reformatted materials are for demonstrational purposes only. The content, edits and layout were not approved by any authors.
Poster Overview
Interactive Pdf Poster
Poster shown in the layout that would be displayed at a congress. Contains supplemental interactive content. Opens in PDF viewer
View PDF PosterMobile-Friendly HTML Poster
Poster content reformatted and scrollable, optimised for digital viewing
View HTML PosterPlain Language Summary (PLS)
Subject matter of the poster summarized in language to be understood by non-specialist audiences
View PLSMobile-Friendly HTML Poster
INTRODUCTION
-
Centanafadine is an inhibitor of norepinephrine, dopamine, and serotonin re-uptake transporters1 currently under investigation for treatment of attention-deficit/hyperactivity disorder (ADHD).2
-
Objective: The efficacy, safety, and tolerability of centanafadine sustained-release (SR) tablets 200 mg/day or 400 mg/day were assessed vs placebo in adults with ADHD in two Phase 3 trials (Study 1: NCT03605680; Study 2: NCT03605836)
METHODS
Study design
- Study 1 and Study 2 were randomized, double-blind (DB), multicenter, placebo-controlled trials comprising four study periods (Figure 1)
- Eligible subjects (Table 1) in the centanafadine 200 mg/ day and 400 mg/day groups received 200 mg total daily dose (TDD) from DB Day 1. The 400 mg/day dose group escalated to 400 mg on Day 8
Endpoints
- Primary efficacy endpoint: Change from baseline at Day 42 in Adult ADHD Investigator Symptom Rating Scale (AISRS) total score
- Key secondary efficacy endpoint: Change from baseline at Day 42 in Clinical Global Impression-Severity of Illness Scale (CGI-S) score
- Safety assessments included the following: Adverse events (AEs); clinical laboratory tests; physical examinations; vital signs; electrocardiograms; Study Medication Withdrawal Questionnaire; and Columbia-Suicide Severity Rating Scale
Statistical analysis
- Efficacy analyses included all randomized subjects who received ≥1 dose of centanafadine or placebo and had both baseline and ≥1 post-randomization efficacy evaluation in the DB period
- Safety analyses included any randomized subject who received ≥1 dose of centanafadine or placebo during the DB period
Figure 1. Design for Centanafadine Study 1 and Study 2 in Adults With ADHD
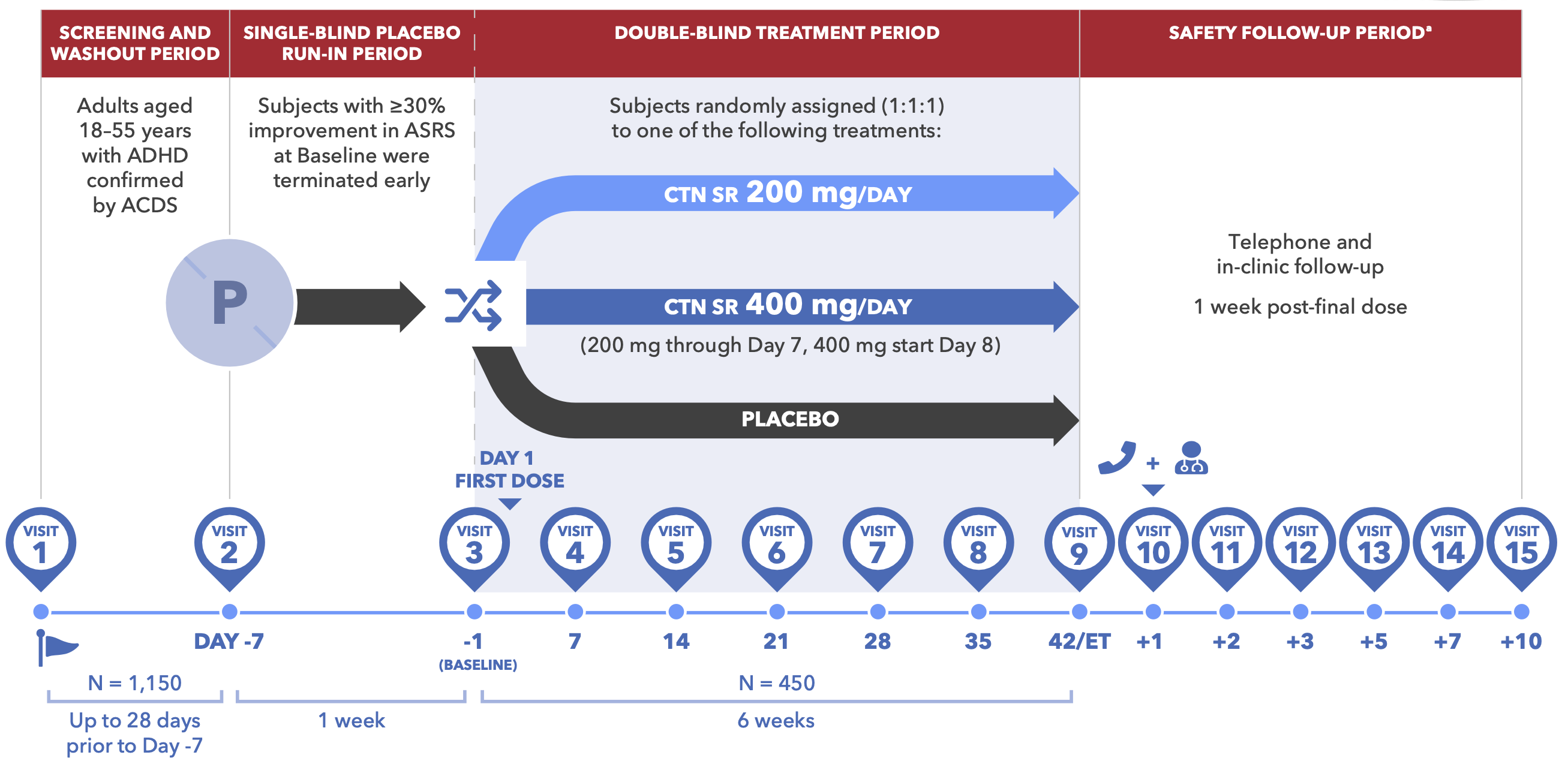
Table 1. Key Inclusion/Exclusion Criteria for Centanafadine Study 1 and Study 2
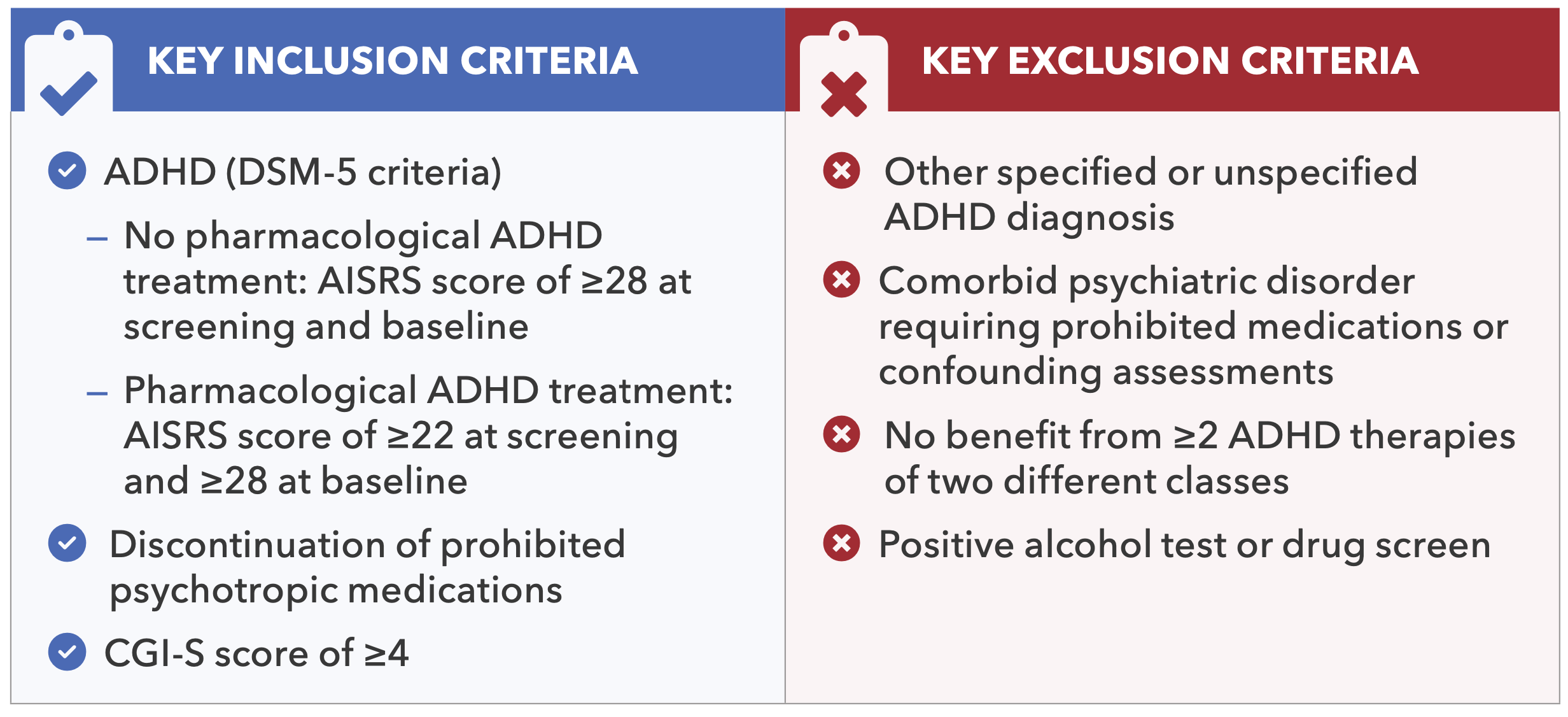
Figure 2. CONSORT Flow Diagram for Centanafadine Study 1 and Study 2 (Randomized Sample)
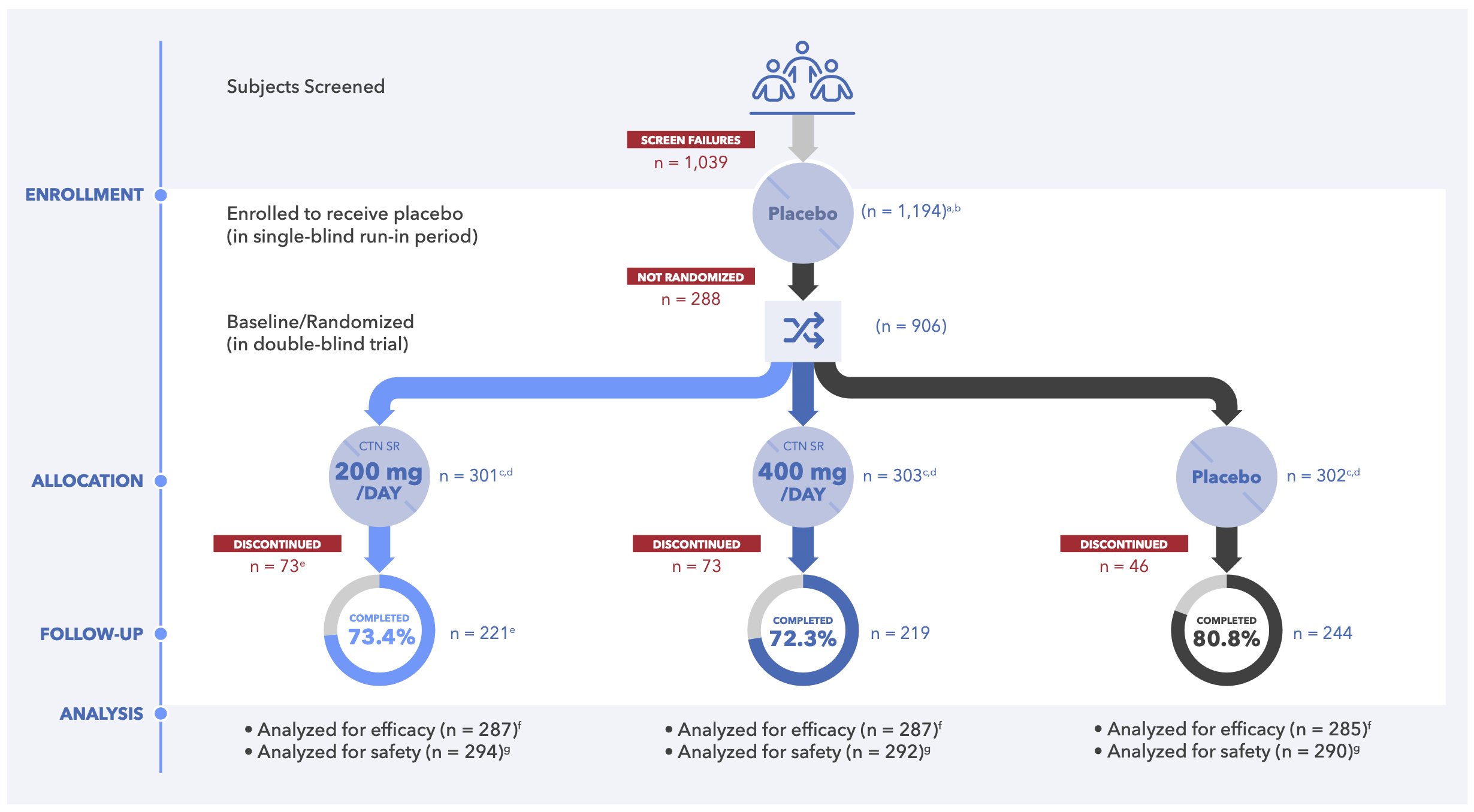
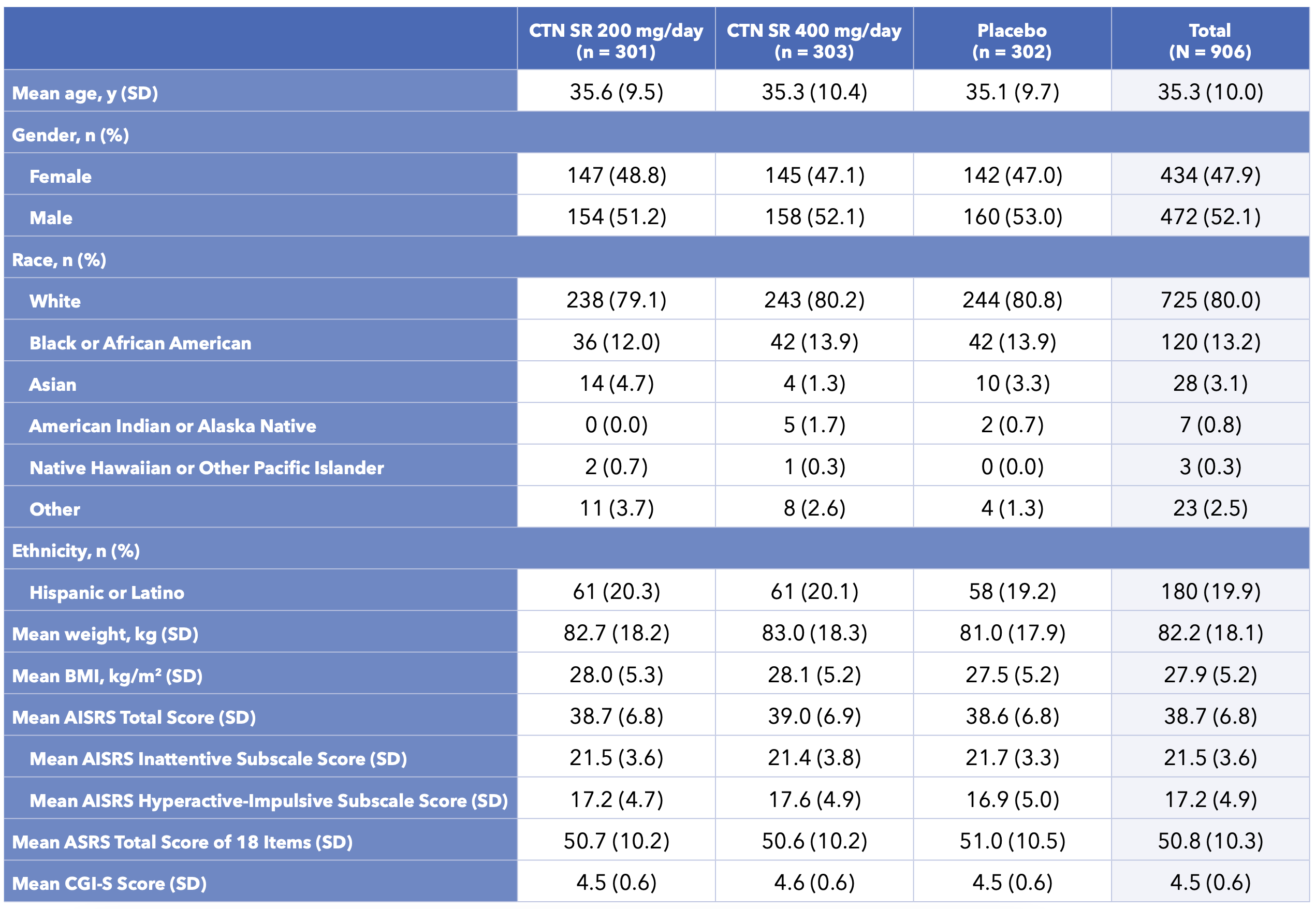
Table 2. Subject Demographics and Baseline Characteristics for Centanafadine Study 1 and Study 2
RESULTS
Subject disposition and baseline demographics (combined results)
- 906 subjects were randomized across both studies (centanafadine 200 mg n = 301; centanafadine 400 mg n = 303; placebo n = 302; Figure 2)
- Baseline demographics and clinical characteristics were balanced between study groups (Table 2)
- Most subjects reported moderate-to-severe ADHD symptoms at baseline (AISRS)
Primary endpoint (efficacy)
- In both studies,the primary endpoint of statistically significant improvement in AISRS total score at Day 42 was achieved for centanafadine 200 mg/day and 400 mg/day compared with placebo (Figures 3 and 4)
- Statistically significant differences in AISRS total scores were seen as soon as Day 7 in Study 1 and Day 28 in Study 2 and were maintained until the end of treatment
Key secondary endpoint (efficacy)
- In both studies, centanafadine 200 mg/day and 400 mg/day demonstrated statistically significant improvements in CGI-S score vs placebo (Figures 5 and 6)
Figure 3. Least Squares Mean Change from Baseline to Day 42 in AISRS Total Score (Primary Endpoint) for Study 1
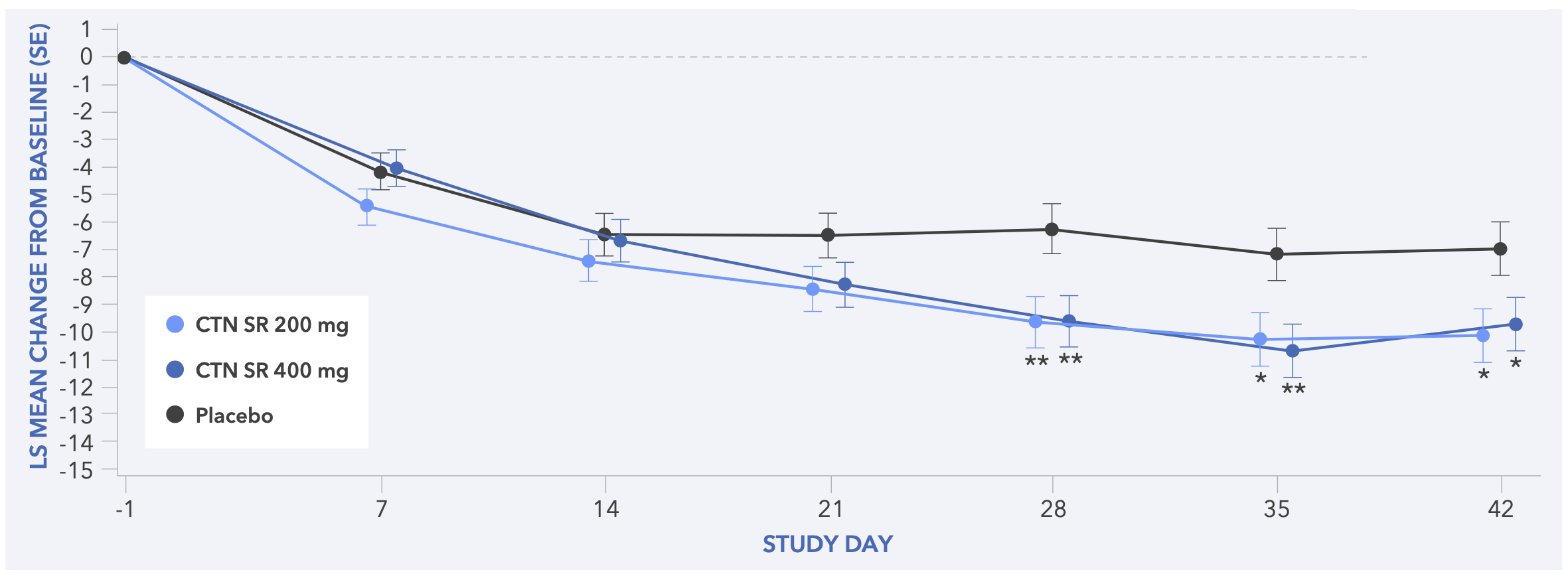
Figure 4. Least Squares Mean Change from Baseline to Day 42 in AISRS Total Score (Primary Endpoint) for Study 2s
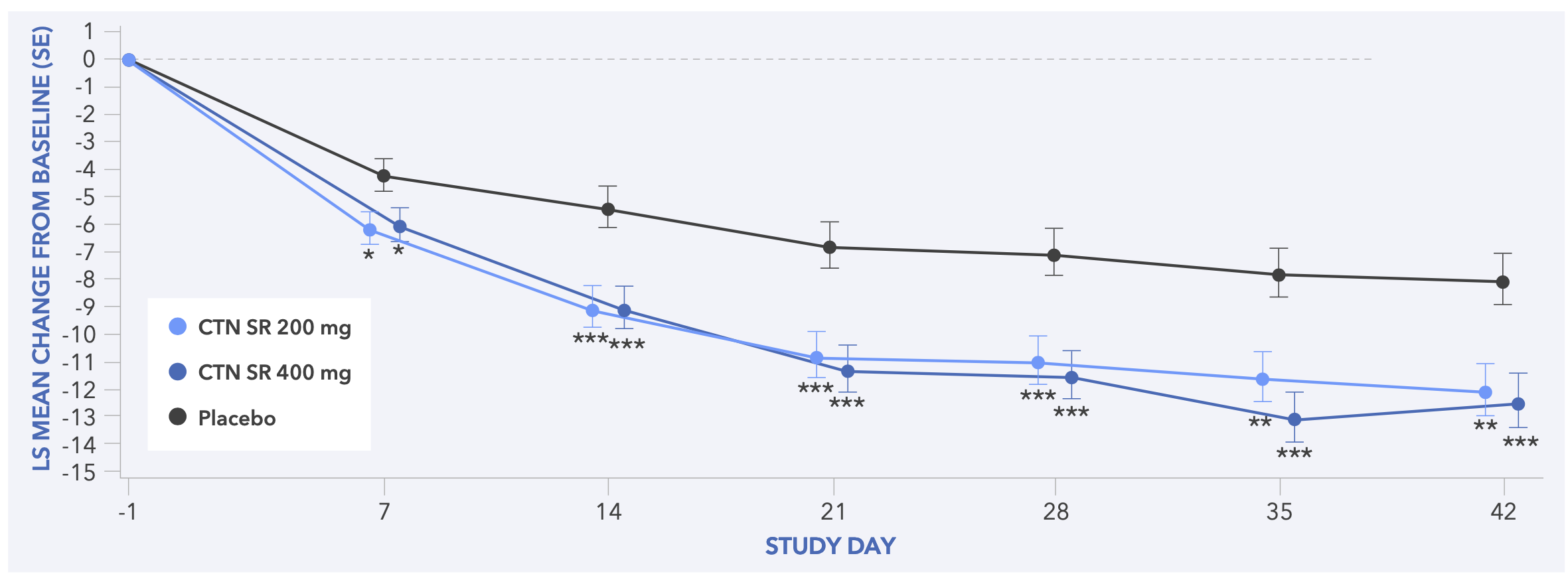
Figure 5. Least Squares Mean Change from Baseline to Day 42 in CGI-S Score (Secondary Endpoint) for Study 1
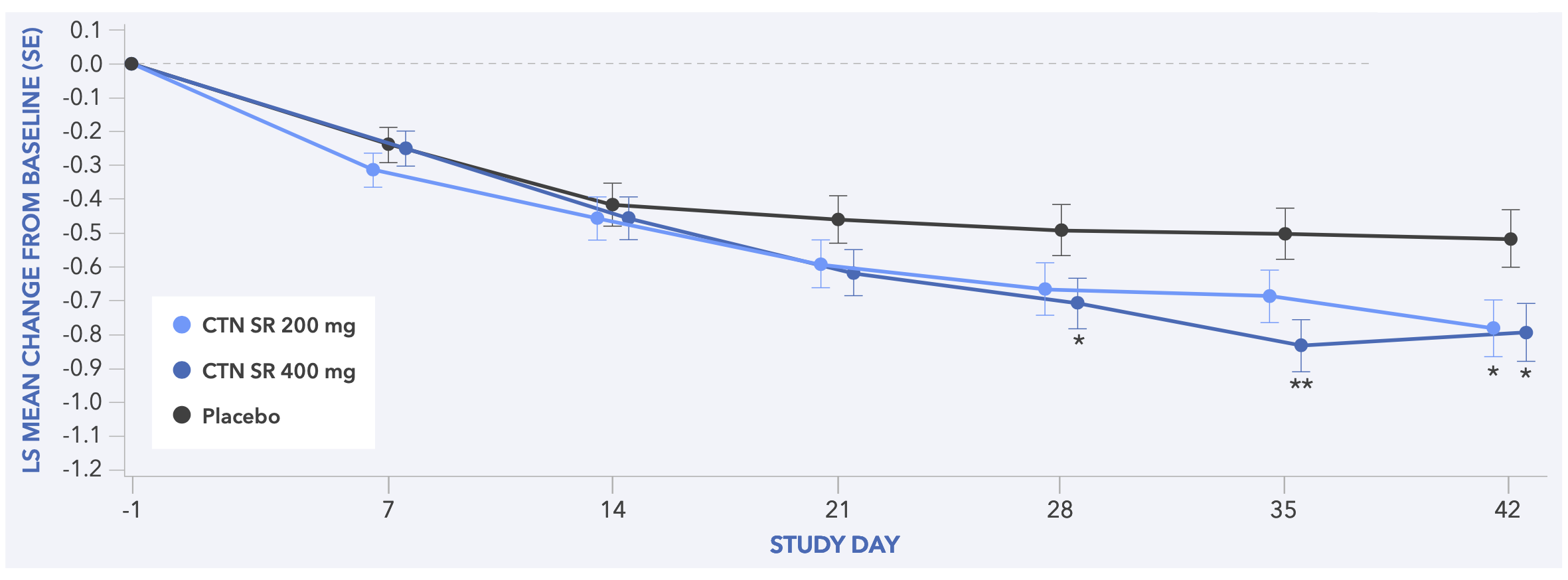
Figure 6. Least Squares Mean Change from Baseline to Day 42 in CGI-S Score (Secondary Endpoint) for Study 2
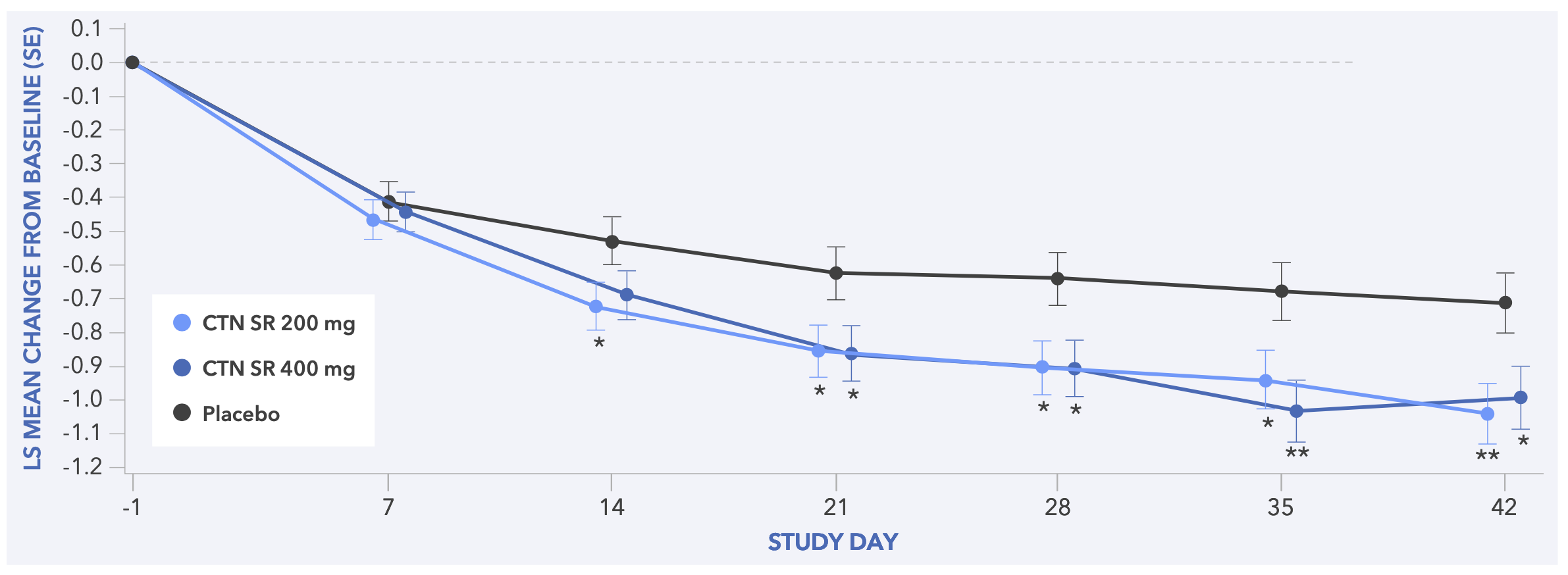
Safety (combined results)
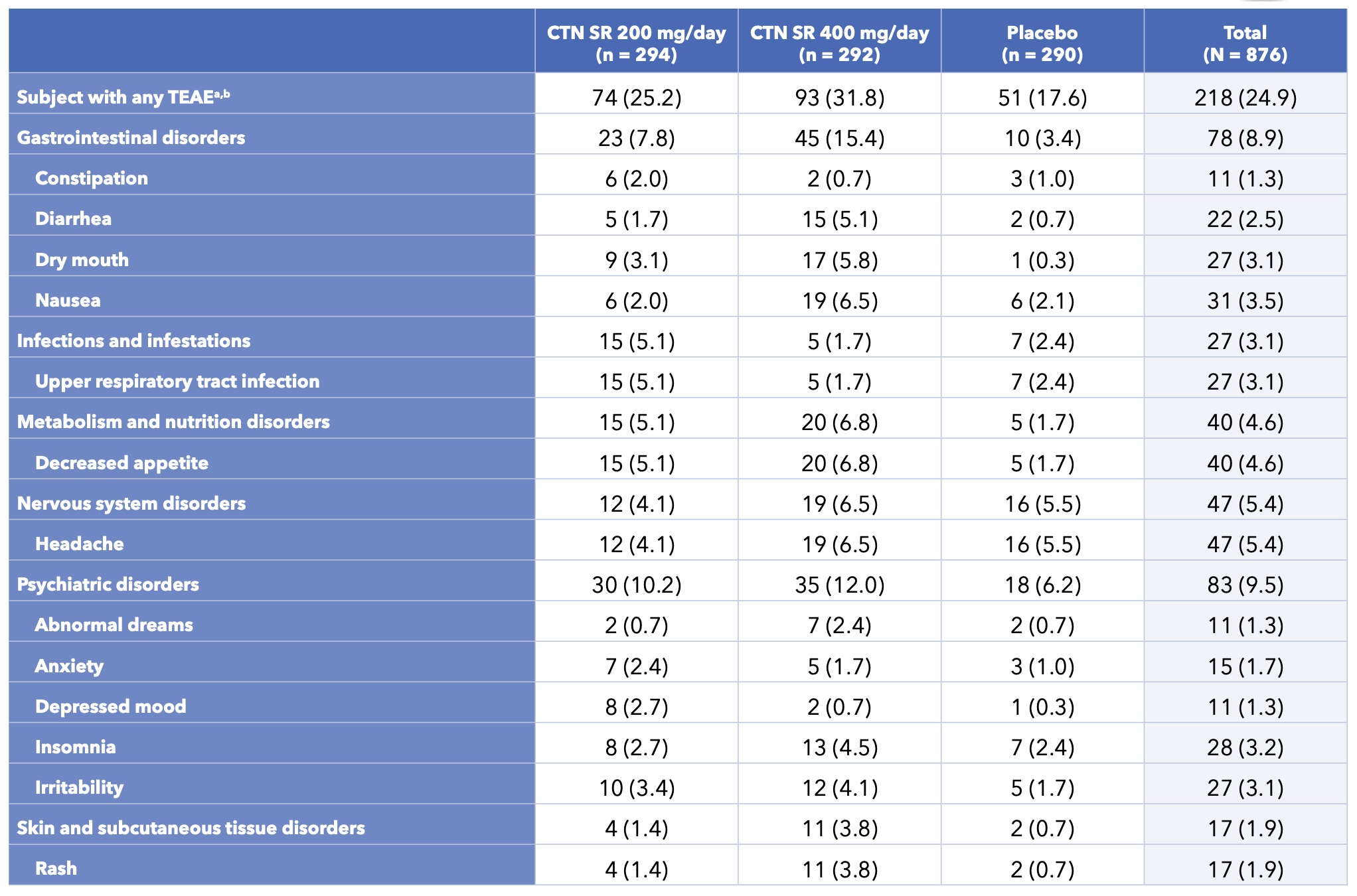
- 738 treatment-emergent adverse events (TEAEs) were experienced by 360 (41.1%) of the 876 subjects who received ≥1 dose of study treatment in the DB period (Table 3)
- The most common TEAEs reported by subjects who received centanafadine were headache and decreased appetite
- Most TEAEs were considered mild or moderate
- No deaths were reported during either study
-
Three subjects reported serious AEs (SAEs; all centanafadine 200 mg/day group); no SAE was considered by investigators to be related to the study drug
- All SAEs resolved and no changes were made to centanafadine treatment
-
Across both studies, 36 subjects (4.1%) discontinued study medication due to TEAEs:
- Centanafadine 200 mg/day: n = 14 (4.8%); centanafadine 400 mg/day: n = 18 (6.2%); placebo: n = 4 (1.4%)
- Reasons for withdrawal due to TEAEs: Psychiatric disorders (n = 12; 1.4%), skin and subcutaneous disorders (n=11;1.3%); rash (n=10;1.1%); and erythematous rash (n = 1; 0.1%)
Table 3. Incidence of TEAEs During the Double-blind Treatment Period Reported by ≥2% in Any Centanafadine Group and Greater Than Placebo in Study 1 and Study 2
Disclosures
Dr. Adler: Received grant and research support from Sunovion Pharmaceuticals, Shire/Takeda Pharmaceuticals, and Otsuka Pharmaceuticals; served as a consultant to Bracket, Sunovion Pharmaceuticals, Shire/Takeda Pharmaceuticals, Otsuka Pharmaceuticals, SUNY, the National Football League, and Major League Baseball; and has received loyalty payments (as inventor) since 2004 from NYU for license of adult ADHD scales and training materials. Drs. Adams, Madera, Hobart, Chang, and McQuade, and Mr. Angelicola: Employees of Otsuka Pharmaceutical Development & Commercialization. Dr. Liebowitz: Otsuka Pharmaceutical Company.
Acknowledgements
We extend our thanks to the patients, their families, and all participating investigators. The two Phase 3 studies presented in this poster were sponsored by Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ. Editorial and production assistance for this poster was provided by BioScience Communications, New York, NY.
References
1. Bymaster FP, et al. Synapse. 2012;66:522-32.
2. Wigal SB, et al. Neuropsychiatr Dis Treat. 2020;16:1411-26.